Physics
Fox
Summary
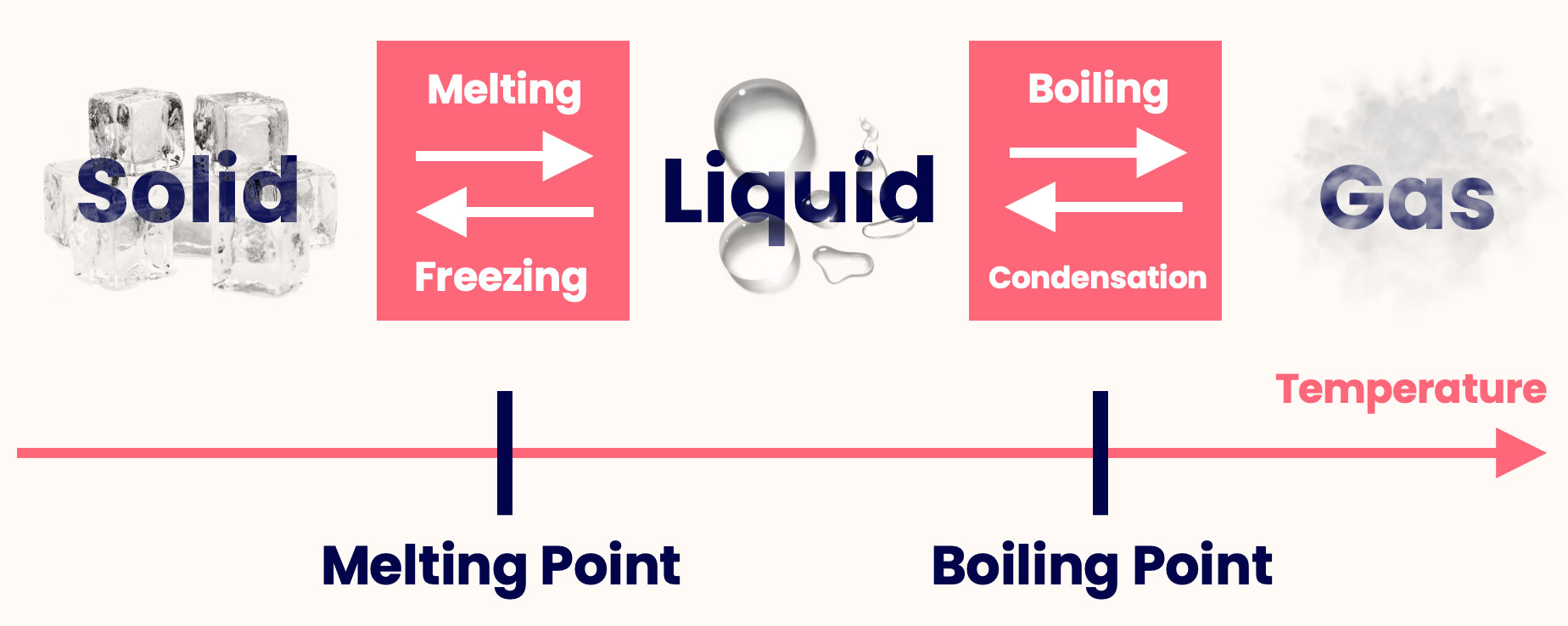
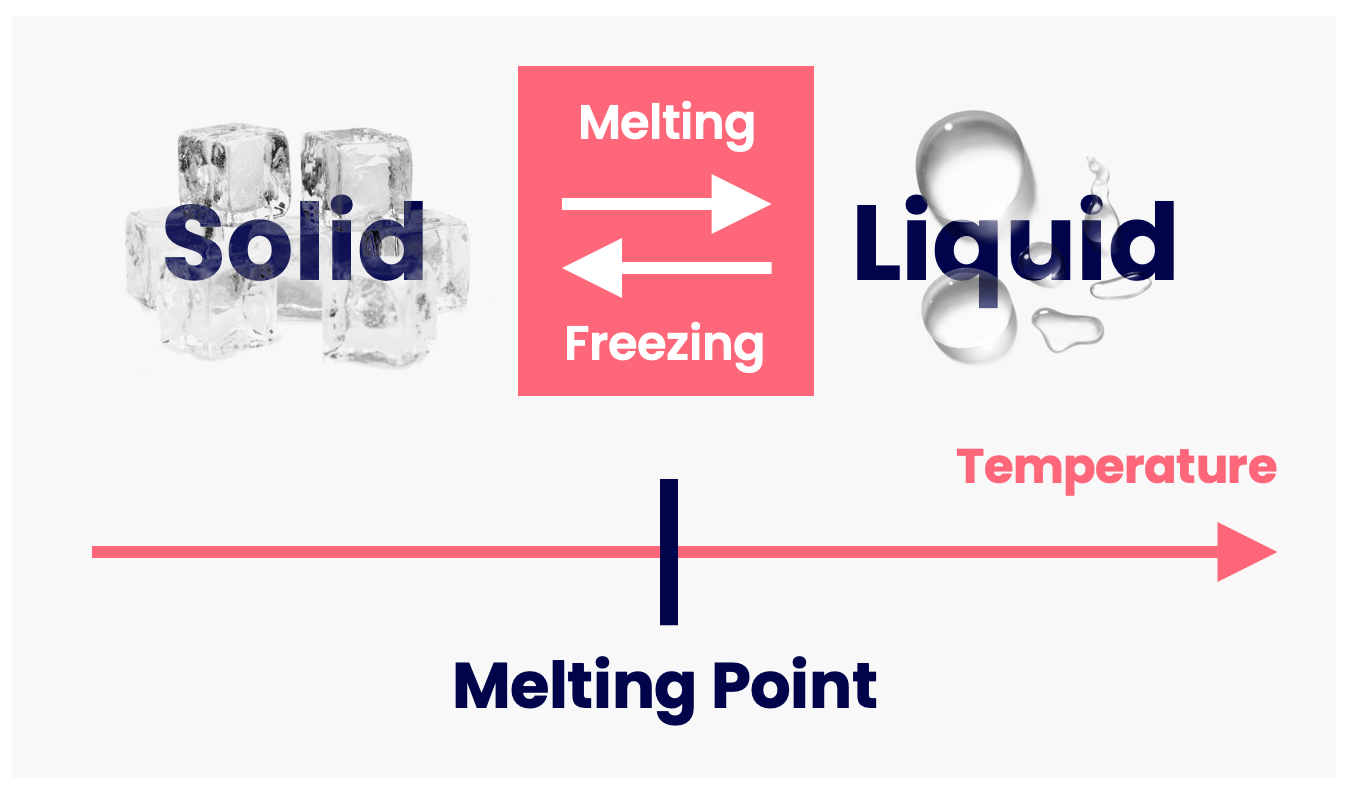
- The transition from solid to liquid is known as melting, and the reverse is freezing.
- The transition from liquid to gas is called boiling, and the reverse is condensation.
- These transitions occur at specific temperatures — the melting point and the boiling point.
I'm at my lowest boiling point
Come help me out
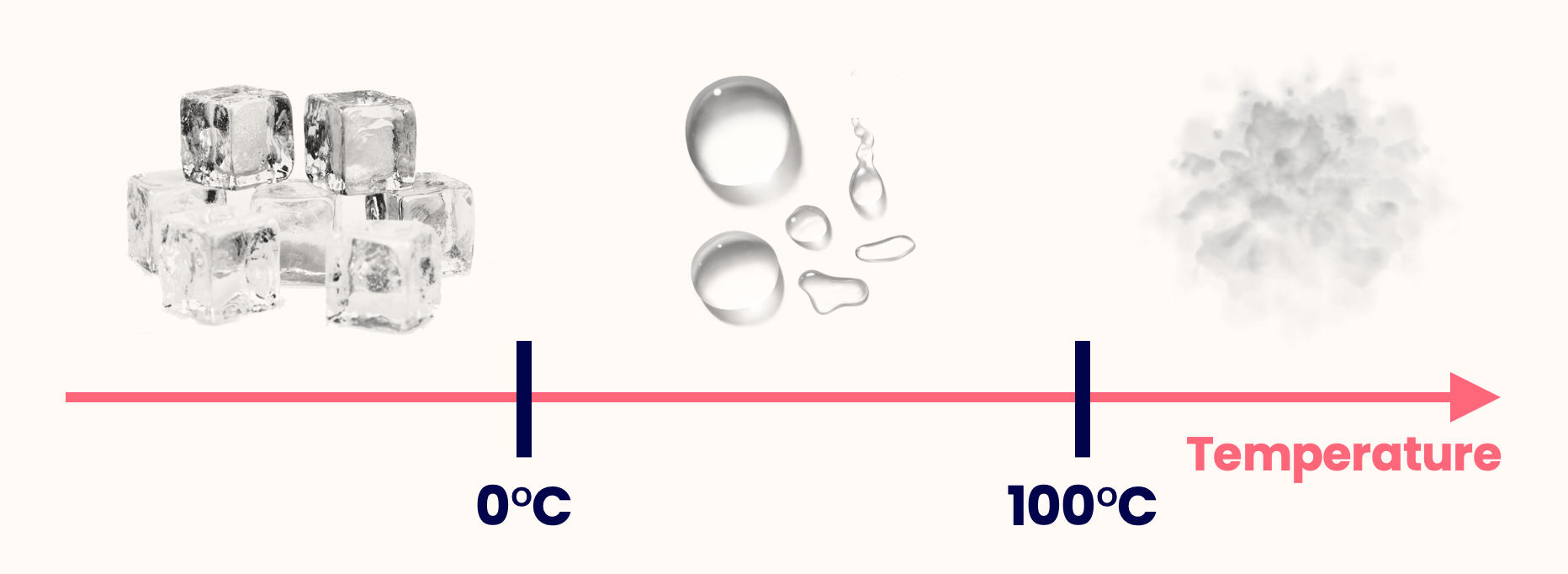
The transition from solid to liquid is known as melting. This occurs at a specific temperature known as the melting point.
The melting point is different for different substances; e.g. ice melts at 0°C, gold at 1064°C, tungsten at 3422°C.

The reverse of melting (i.e. going from liquid to solid) is known as freezing.
Liquids take the shape of any container they're in. Therefore, if you pour a liquid into a container and then freeze it, it's now a solid that keeps the shape of the container. With this process, we can craft things such as gold rings, ice lollies, and gallium lego figures.
The transition from liquid to gas is known as boiling. This occurs at a specific temperature known as the boiling point.
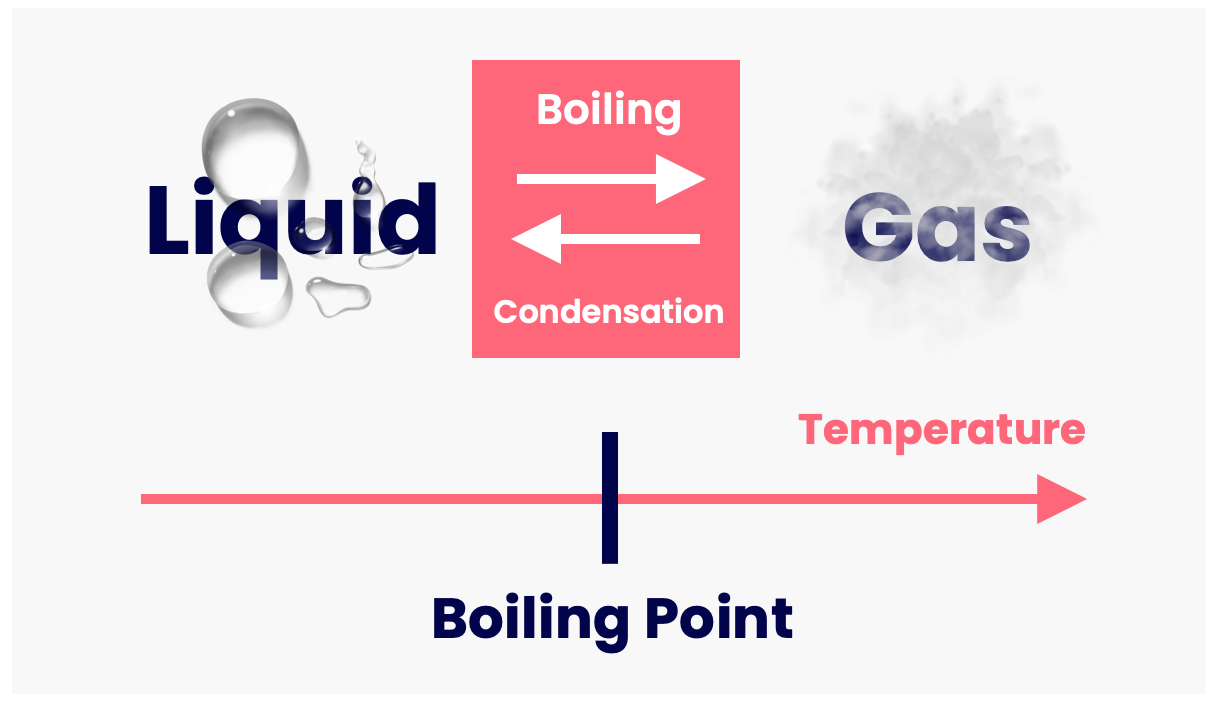
The boiling point is different for different substances — e.g. water boils at 100°C, gold at 2970°C, tungsten at 5930°C.
The reverse of melting (i.e. going from gas to liquid) is known as condensation.
Boiling and condensation are present whenever a kettle is used. The kettle boils water to form steam, and the steam condenses back to water when it touches a nearby surface (because the surface is much colder than the steam).

Here's something most people don't know: steam is invisible. The ‘steam’ that you see is actually tiny droplets of water, because some of the steam has condensed in the air.
It is not a coincidence that water melts at exactly 0°C, and boils at exactly 100°C — this is how the °C temperature scale is defined!
Practically all units we use in physics are invented by people, and °C is no exception.
When the temperature of a substance increases, its thermal energy increases too. What is thermal energy? What even is energy in the first place? Let's find out in the next chapter...
Congratulations!
7 of 7 questions completed
+ ⭐️ collected.
Sign up (for free!) to:
• save your progress 📊
• create constellations✨
• customise your fox! 🦊